Compound degraded:Aldrin
General Description (About POP compound)
Aldrin is a synthetic organochlorine pesticide, and was used as a broad-spectrum soil insecticide for protection of food crops, and as seed dressing for the control of pests such as ants and termites. It is readily metabolized to dieldrin in both animals and plants, and therefore aldrin residues are rarely detected in animals, and if so only in small amounts
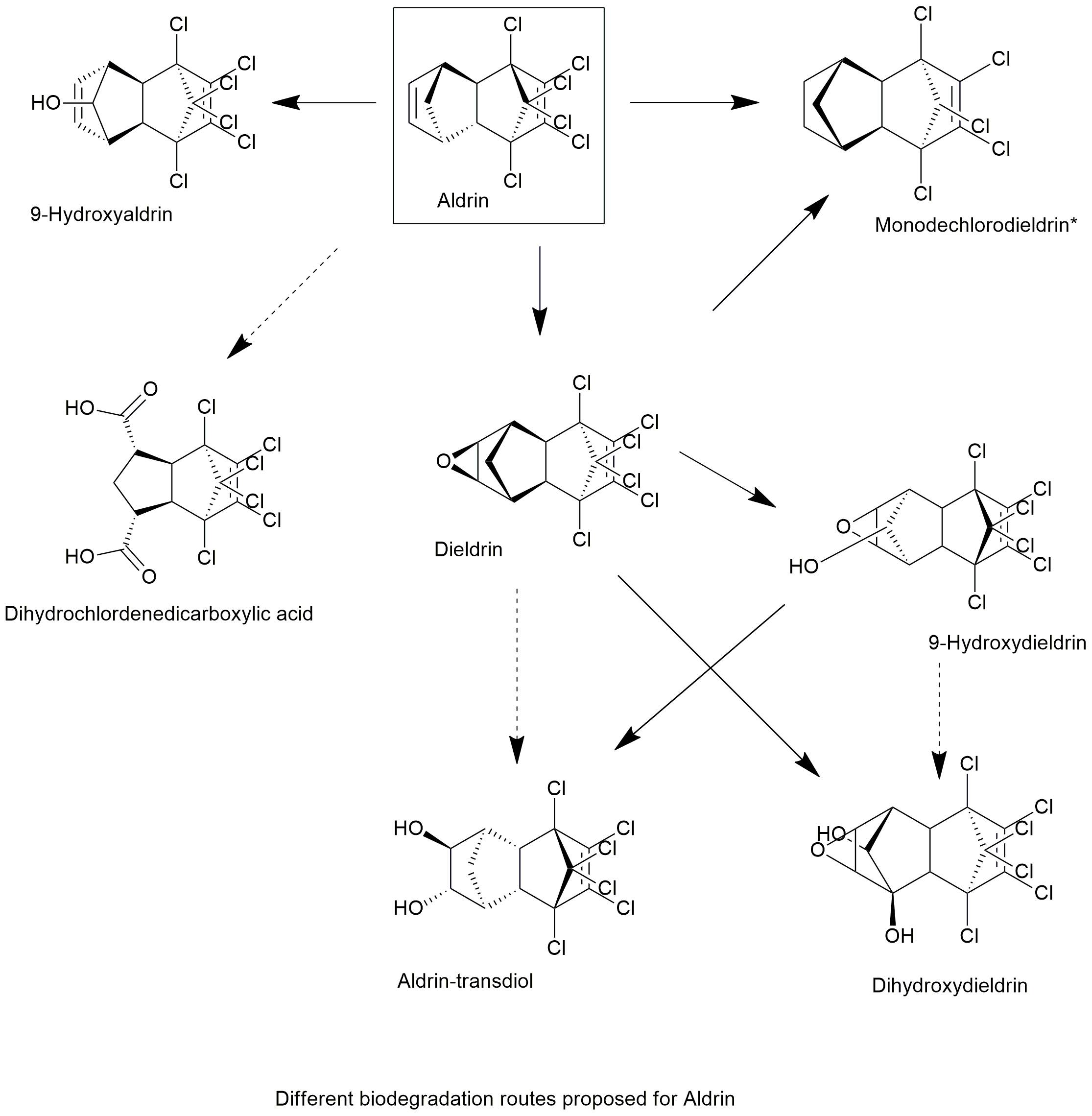
Biodegradation pathway
Publications
| Abstract | Title | Authors | Article Link |
|---|---|---|---|
| Aldrin and its metabolite dieldrin are persistent organic pollutants that contaminate soil in many parts of the world. Given the potential hazards associated with these pollutants, an efficient degradation method is required. In this study, we investigated the ability of Pleurotus ostreatus to transform aldrin as well as dieldrin in pure liquid cultures. This fungus completely eliminated aldrin in potato dextrose broth (PDB) medium during a 14-day incubation period. Dieldrin was detected as the main metabolite, and 9-hydroxylaldrin and 9-hydroxyldieldrin were less abundant metabolites. The proposed route of aldrin biotransformation is initial metabolism by epoxidation, followed by hydroxylation. The fungus was also capable of degrading dieldrin, a recalcitrant metabolite of aldrin. Approximately 3, 9, and 18% of dieldrin were eliminated by P. ostreatus in low-nitrogen, high-nitrogen, and PDB media, respectively, during a 14-day incubation period. 9-Dihydroxydieldrin was detected as a metabolite in the PDB culture, suggesting that the hydroxylation reaction occurred in the epoxide ring. These results indicate that P. ostreatus has potential applications in the transformation of aldrin as well as dieldrin. | Biodegradation of Aldrin and Dieldrin by the White-Rot Fungus Pleurotus ostreatus | Purnomo et al., 2017 | Link |
| Degradation of aldrin (1,2,3,4,10,10-Hexachloro-1,4,4a,5,8,8a-hexahydro-1,4:5-8-dimethanonaphthalene), heptachlor (1H-1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro-4,7-methano indene), dieldrin (1a?,2?,2a?,3?,6?,6a?,7?,7a?)-3,4,5,6,9,9-Hexachloro-1a,2,2a,3,6,6a,7,7a-octahydro-2,7:3,6-d-methanonaphtha[2,3-b]oxirene, and heptachlor epoxide (1a?, 1b?,2?,5?,5??,6?,6a?-2,3,4,5,6,7,7-Heptachloro-1a,1b,5,5a,6,6a-hexahydro-2,5-methano-2H-inden[1,2-b]-oxirene) was tested using free cultures of Pseudomonas fluorescens under controlled conditions. Pesticide concentrations were monitored by gas chromatography during 120 h. Percentages of degradation and biodegradation rates (BDR) were calculated. Data showed a trend suggesting a relation between chemical structure and degradability. Degradation kinetics for each pesticide tested showed that the highest degradation rates were found in the first 24 h. Kinetics data were adjusted to an empirical equation in order to predict their behavior, and the correlation coefficients obtained were satisfactory. Gas chromatography/mass spectrometry (GC/MS) analysis of the final extracts allowed the identification of chlordene and monodechlorodieldrin, which have been reported as final metabolite produced in the biodegradation of this kind of compounds. Regarding adsorption of pesticides on activated vegetal carbon, we concluded that removal efficiencies between 95.45 and 97.18% can be reached, depending on the pesticide and the carbon dose applied. The values for K from the Freundlich equation were quite similar for the four pesticides (between 1.0001 and 1.04), whereas the n values were quite different for each pesticide in the following order of affinity: dieldrin > aldrin > heptachlor epoxide > heptachlor. Equilibrium times, very important for scaling up the process, were between 43 min and 1 h, for the heptachlor epoxide and the heptachlor, respectively. | Removal of Aldrin, Dieldrin, Heptachlor, and Heptachlor Epoxide Using Activated Carbon and/or Pseudomonas fluorescens Free Cell Cultures | Bandala et al., 2007 | Link |
| White rot fungi can degrade a wide spectrum of recalcitrant organic pollutants, including polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated biphenyls (PCBs). In this experiment, 20 white rot fungi, belonging to genus Phlebia, were investigated for their ability to degrade dieldrin. Based on the screening results, we further investigated Phlebia acanthocystis, Phlebia brevispora, and Phlebia aurea to determine their degradation capacity and metabolic products towards dieldrin and aldrin. The three fungi were able to remove over 50% of dieldrin in a low nitrogen medium, after 42 d of incubation. Three hydroxylated products were detected as metabolites of dieldrin, suggesting that in Phlebia strains, hydroxylation reactions might play an important role in the metabolism of dieldrin. In contrast to dieldrin, aldrin exhibited higher levels of degradation activity. Over 90% of aldrin was removed after 28 d of incubation, and several new metabolites of aldrin in microorganisms, including 9-hydroxyaldrin and two carboxylic acid products, were detected in fungal cultures. These results indicate that the methylene moiety of aldrin and dieldrin molecules might be prone to enzymatic attack by white rot fungi. In this study, we describe for the first time a new metabolic pathway of both compounds by fungi of genus Phlebia. | Novel metabolic pathways of organochlorine pesticides dieldrin and aldrin by the white rot fungi of the genus Phlebia | Xiao et al., 2011 | Link |
| An aerobic dieldrin-degrading fungus, Mucor racemosus strain DDF, and two aerobic endosulfan-degrading fungal strains, Mortierella sp. strains W8 and Cm1-45, were isolated from soil contaminated with organochlorine pesticides. Strain DDF degraded more than 90% dieldrin during 10-days of incubation at 25°C and showed the production of a small amount of aldrin trans-diol. Moreover, strain DDF reduced levels of aldrin trans-diol while producing unknown metabolites that were determined to be aldrin trans-diol exo- and endo-phosphates. On the other hand, Mortierella sp. strains W8 and Cm1-45 degraded more than 70% and 50% of ? and ?-endosulfan, respectively, over 28 days at 25°C, in liquid cultures containing initial concentrations of 8.2?µM of each substance. Only a small amount of endosulfan sulfate, a persistent metabolite, was detected in the both cultures, while these strains could not degrade endosulfan sulfate when this compound was provided as the initial substrate. Both strains generate endosulfan diol as a first step in the degradation of endosulfan, then undergo further conversion to endosulfan lactone. | Biodegradability and biodegradation pathways of chlorinated cyclodiene insecticides by soil fungi | Kataoka. 2018 | Link |
| This study was the first to evaluate the occurrence, residue levels, spatial distribution and sources of DDT and other Persistence Organic Pollutants (POPs), which can be found in the Nyabarongo lower catchment (NLC) in Rwanda. These include Aldrin, Dieldrin, Endosulfan, Endrin, Hexachlorocyclohexane (HCH), Heptachlor, Heptachlorepoxide, Hexachlorobenzene (HCB), Isodrin, Methoxychlor, Mirex and Polychlorinated biphenyls (PCBs). A total of 108 soil samples were collected in the wetland area, both extracted and eluted with cyclohexane and analysed by GC-MS. The results indicated that DDT isomers and degradation products were major POPs and were detected in 44 samples (40%). Their detection frequency followed the order of 4,4?-DDE > 4,4' -DDT > 4,4' -DDD > 2,4' -DDT > 2,4' -DDD and 2,4' -DDE. Residues varied from non-detected (nd) to 120 ?g kg?1 dry weight (dw), with a mean value of 3.93 ?g kg?1 dw and a high variation (SD = 10.17 ?g kg?1 dw). The degradation ratios confirmed both the historical and recent application of DDT and Dieldrin (0.53–18 ?g kg?1 dw). Other detected POPs included PCBs in Kigali city which ranged from 0.1 to 0.21 ?g kg?1 dw, confirming that the old contamination drifted from electric transformers. Aldrin (0.38–0.59 ?g kg?1 dw); Heptachlor (0.14–0.19 ?g kg?1 dw) residues probably reached the catchment through rain-washout. This study confirms that even though Rwanda banned the use of DDT and other POPs including pesticides (Aldrine, Chlordane, DDT, Dieldrine, Endrine, Heptachlor, Hexachlorobenzene, Mirex, and Toxaphene); Industrial products (Hexachlorobenzene and Polychlorobiphenyl PCBS) and unintentional sub-products, since 2002, some of above products are still used in random areas (e.g: DDT, Dieldrin). The highest residues were detected close to Lake Muhazi and areas surrounding Kigali city. This study recommends full evaluation of human health and ecological risks from exposure to DDT. Additionally, the National Implementation Plan (NIP) for the Stockholm Convention to eliminate POPs should be reinforcement through strengthening the market control and educational programs. | First evaluation of DDT (dichlorodiphenyltrichloroethane) residues and other Persistence Organic Pollutants in soils of Rwanda: Nyabarongo urban versus rural wetlands | Umulisa et al., 2020 | Link |
| Dieldrin is one of the most persistent organic pollutants, and its oxidative degradation pathways by aerobic microorganisms to 6,7-trans-dihydroxydihydroaldrin (otherwise known as aldrin-trans-diol) and 9-hydroxydieldrin are well documented. The dieldrin-degrading fungus, Mucor racemosus strain DDF, can decrease dieldrin levels with simultaneous production of a small amount of aldrin-trans-diol. A reduction in the levels of aldrin-trans-diol by strain DDF has also been observed. Based on these results, it has been suggested that strain DDF transforms dieldrin to more polar compounds via aldrin-trans-diol. We have conducted a study to identify the metabolites arising from aldrin-trans-diol. The results showed that strain DDF gave reduced levels of aldrin-trans-diol and also produced unknown metabolites. Ultra performance liquid chromatography-electrospray ionization-mass spectroscopy (UPLC-ESI-MS) analysis indicated the metabolites to be either sulfated- or phosphorylated- derivatives of aldrin-trans-diol, but with the metabolites retaining six chlorine atoms. Therefore, the candidate derivatives were synthesized and the retention times of the natural metabolite and the synthetic phosphate were compared. As a result of a co-injection experiment, the metabolites were determined to be aldrin-trans-diol exo- and endo-phosphates. These results were also supported by high-resolution-fast atom bombardment-mass spectrometry (HR-FAB-MS) of the natural metabolite (? = 0.63 ppm). Phosphorylation of aldrin-trans-diol is the first reported example of phosphate conjugation in microorganisms. | Novel phosphorylation of aldrin-trans-diol by dieldrin-degrading fungus Mucor racemosus strain DDF | Yamakazi et al., 2014 | Link |
