Compound degraded:Polychlorinated dibenzo-p-dioxins (PCDDs)
General Description (About POP compound)
Polychlorinated dibenzo-p-dioxins (PCDDs) commonly known as dioxins (PCDDs), are toxic environmental pollutants formed from various sources. They are highly lipophilic, sparingly soluble in water, and bio, physical and chemically stable. The most toxic congener is 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD), one of the most toxic molecules known so far, with mutagenic, teratogenic and carcinogenic characteristics. In certain laboratory animals and wildlife species, dioxin can cause death following even tiny doses, leading TCDD to be called “the most toxic man-made chemical.” Elimination of these pollutants from the environment is a difficult task due to their persistent and ubiquitous nature. Removal of dioxins by biological degradation (biodegradation) is considered a feasible method as an alternative to other expensive physicochemical approaches.
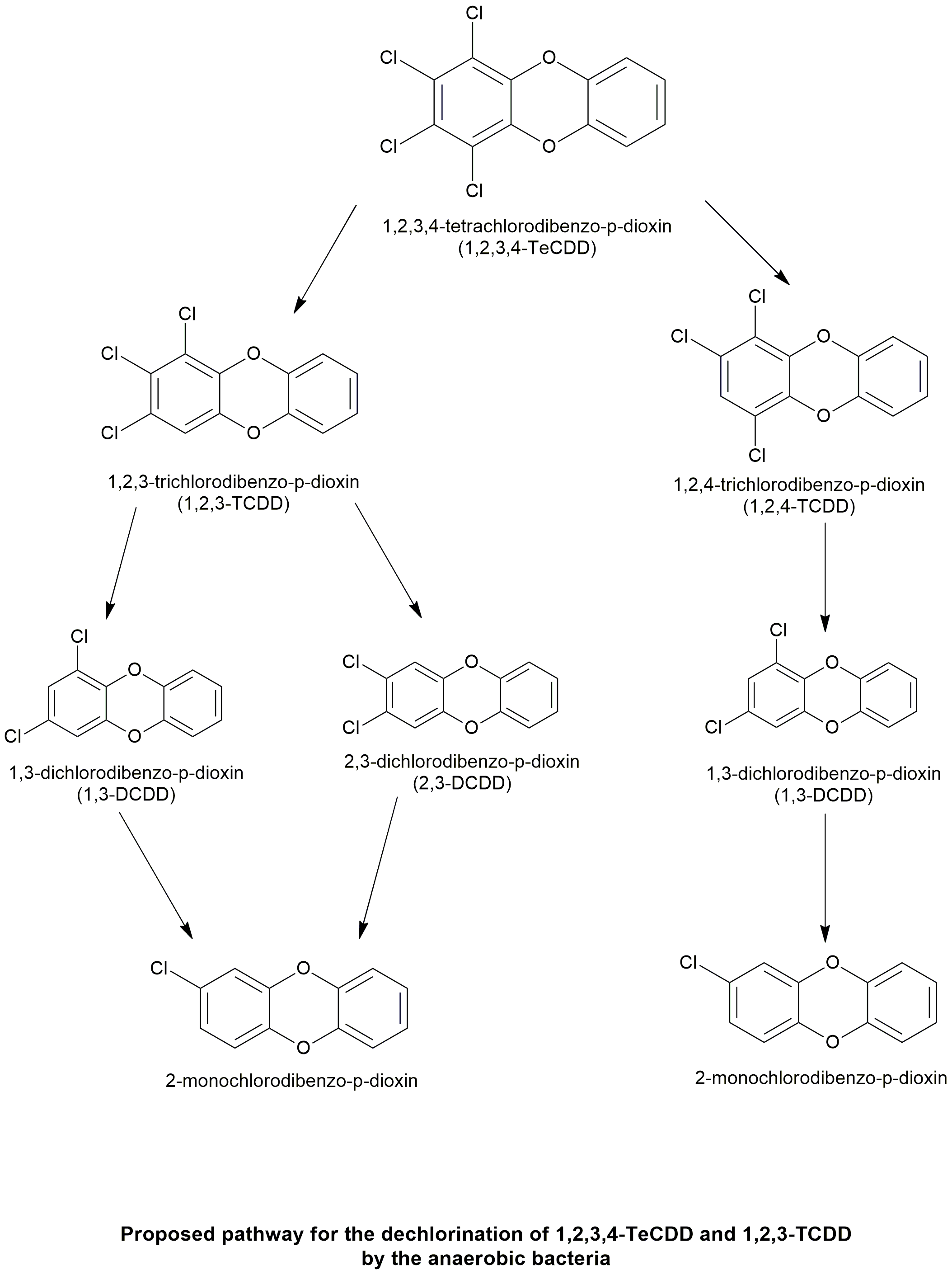
Biodegradation pathway
Publications
| Abstract | Title | Authors | Article Link |
|---|---|---|---|
| This review article summarizes what is known about human health following exposure to dioxins. It is meant primarily for health professionals but was also written with the general public in mind. The need for such an article became apparent to the authors following media inquiries at the time the then Ukraine presidential candidate Victor Yushchenko was deliberately poisoned with the most toxic dioxin, tetrachlorodibenzodioxin or TCDD. | Dioxins: An overview | Schecter et al., 2006 | Link |
| Dioxins are a class of extremely hazardous molecules that might pose a threat to the environment. This work evaluated the microbial degradation of 1,2,3,4-tetrachlorodibenzo-p-dioxin (1,2,3,4-TCDD), in liquid broth using three brown-rot fungi and one white-rot fungi as control. A fast and reliable extraction method with recoveries of over 98% together with a validated GC–MS method was developed, and applied to quantify 1,2,3,4-TCDD in liquid broth, mycelia and reaction flask, with detection limits of 10 ppb. Among the four strains tested, brown-rot fungus Aspergillus aculeatus showed best results, removing up to 21% of dioxin after 30-day incubation. The results open both a path for biotechnological interest in bioremediation purposes and environmental behavior studies by using brown-rot fungus. | Biodegradation of 1,2,3,4-tetrachlorodibenzo-p-dioxin in liquid broth by brown-rot fungi | Perlatti et al., 2013 | Link |
| Anaerobic enrichment cultures derived from contaminated Kymijoki River sediments dechlorinated 1,2,3,4-tetrachlorodibenzofuran (1,2,3,4-tetra-CDF), octachlorodibenzofuran (octa-CDF) and 1,2,3,4-tetrachlorodibenzo-p-dioxin (1,2,3,4-tetra-CDD). 1,2,3,4-tetra-CDF was dechlorinated via 1,2,3-, 2,3,4-, and 1,3,4/1,2,4-tri-CDFs to 1,3-, 2,3-, and 2,4-di-CDFs and finally to 4-mono-CDF. The dechlorination rate of 1,2,3,4-tetra-CDF was generally slower than that of 1,2,3,4-tetra-CDD. The rate and extent of 1,2,3,4-tetra-CDD dechlorination was enhanced by addition of pentachloronitrobenzene (PCNB) as a co-substrate. Dechlorination of spiked octa-CDF was observed with the production of hepta-, hexa-, penta- and tetra-CDFs over 6 months. Two major phylotypes of the Chloroflexi community showed an increase, one of which was identical to the Dehalococcoides mccartyi Pinellas subgroup. A set of twelve putative reductive dehalogenase (rdh) genes increased in abundance with addition of 1,2,3,4-tetra-CDF, 1,2,3,4-tetra-CDD and/or PCNB. This information will aid in understanding how indigenous microbial communities impact the fate of PCDFs and in developing strategies for bioremediation of PCDD/F contaminated sediments. | Enriching for microbial reductive dechlorination of polychlorinated dibenzo-p-dioxins and dibenzofurans | Liu et al., 2014 | Link |
| A suite of experiments were conducted to ascertain whether dehalogenation of a model dioxin compound could be stimulated in marine sediments by supplementation with halogenated analogues to enrich for dehalogenating bacteria and if growth by members of the Chloroflexi-like group was associated with dioxin removal. Five halogenated compounds (tetrachlorobenzene, tetrachloroanisole, tetrachlorophenol, tetrachlorobenzoic acid and trichloroacetophenone) were added with 1,2,3,4-tetrachlorodibenzo-p-dioxin (TeCDD) to estuarine sediments from four sites in San Diego Bay and the coast of southern New Jersey to test for dioxin dehalogenation. Most of the halogenated additives were found to stimulate dechlorination of the model dioxin. Molecular analysis of the bacterial population using 16S rRNA and reductive dehalogenase genes indicated that distinct microbial populations were enriched with each halogenated co-amendment. Additionally, Chloroflexi-like ribosomal genes associated with dehalogenation were detected. For example, quantitative real-time PCR analysis of 16S rRNA and reductive dehalogenase gene copy number in the microcosms showed a positive correlation with 1,2,3,4-TeCDD reductive dechlorination in coastal sediments amended with different halogenated additives. These results suggest that specific Chloroflexi-like microorganisms related to Dehalococcoides are involved in 1,2,3,4-TeCDD reductive dechlorination. | Comparison of anaerobic microbial communities from Estuarine sediments amended with halogenated compounds to enhance dechlorination of 1,2,3,4-tetrachlorodibenzo-p-dioxin | Ahn et al., 2007 | Link |
| Halogenated coamendments enhanced dechlorination of 31 microM of spiked 1,2,3,4-tetrachlorodibenzo-p-dioxin (TeCDD) and 49 microM of spiked 1,2,3,4-tetrachlorodibenzofuran (TeCDF) in sediments from San Diego Bay (CA, USA) and Tuckerton (NJ, USA). Dechlorination of 1,2,3,4-TeCDD occurred to a greater extent under methanogenic than under sulfate-reducing conditions. The most effective stimulation of 1,2,3,4-TeCDD dechlorination occurred with coamendment of 25 microM of 1,2,3,4-tetrachlorobenzene (TeCB), 2,3,4,5-tetrachloroanisole (TeCA), 2,3,4,5-tetrachlorophenol, or 2',3',4'-trichloroacetophenone plus 500 microM lactate and 500 microM propionate as electron donors. The 1,2,3,4-TeCDD dechlorination was evident after three months and sequentially produced mainly 1,2,4-trichlorodibenzo-p-dioxin, 1,3-dichlorodibenzo-p-dioxin, and 2-monochlorodibenzo-p-dioxin (MCDD). Monobromophenols (2-bromo-, 3-bromo-, and 4-bromophenol), monochlorophenols (2-chloro-, 3-chloro-, and 4-chlorophenol), 2,3,5,6-tetrachlorobenzoate, or electron donors alone stimulated less 1,2,3,4-TeCDD dechlorination, with activity apparent only after six months. The 1,2,3,4-TeCDD dechlorination produced 50 mol % 2-MCDD after six months in sediments from the more contaminated Graving Dock and Paleta Creek sites in San Diego Bay. The 1,2,3,4-TeCDD dechlorination by sediments from the less contaminated Shelter Island site in San Diego Bay and in pristine Tuckerton sediments did not produce 2-MCDD. Dechlorination of 1,2,3,4-TeCDF to tri- and dichlorinated daughter products was significantly enhanced by TeCB and TeCA. These results suggest that halogenated aromatic compounds with structural similarity to 1,2,3,4-TeCDD/F stimulate bacteria with the ability to dechlorinate chlorinated dibenzo-p-dioxin and furans. | Co-amendment with halogenated compounds enhances anaerobic microbial dechlorination of 1,2,3,4-tetrachlorodibenzo-P-dioxin and 1,2,3,4-tetrachlorodibenzofuran in estuarine sediments | Ahn et al., 2005 | Link |
| Dehalococcoides ethenogenes strain 195 dechlorinates tetrachloroethene to vinyl chloride and ethene, and its genome has been found to contain up to 17 putative dehalogenase gene homologues, suggesting diverse dehalogenation ability. We amended pure or mixed cultures containing D. ethenogenes strain 195 with 1,2,3,4-tetrachlorodibenzo-p-dioxin, 2,3,7,8-tetrachlorodibenzo-p-dioxin, 2,3-dichlorodibenzo-p-dioxin, 1,2,3,4-tetrachloro-dibenzofuran, 2,3,4,5,6-pentachlorobiphenyl, 1,2,3,4-tetrachloronaphthalene, various chlorobenzenes, or a mixture of 2-, 3-, and 4-chlorophenol to determine the dehalogenation ability. D. ethenogenes strain 195 dechlorinated 1,2,3,4-tetrachlorodibenzo-p-dioxin to a mixture of 1,2,4-trichlorodibenzo-p-dioxin and 1,3-dichlorodibenzo-p-dioxin. 2,3,4,5,6- Pentachlorobiphenyl was dechlorinated to 2,3,4,6- and/or 2,3,5,6-tetrachlorobiphenyl and 2,4,6-trichlorobiphenyl. 1,2,3,4-Tetrachloronaphthalene was dechlorinated primarily to an unidentified dichloro-naphthalene congener. The predominant end products from hexachlorobenzene dechlorination were 1,2,3,5-tetrachloro-benzene and 1,3,5-trichlorobenzene. We did not detect dechlorination daughter products from monochlorophenols, 2,3-dichlorodibenzo-p-dioxin or 2,3,7,8- tetrachlorodibenzo-p-dioxin. D. ethenogenes strain 195 has the ability to dechlorinate many different types of chlorinated aromatic compounds, in addition to its known chloroethene respiratory electron acceptors. Remediation of sediments contaminated with aromatic halogenated organic pollutants such as polychlorinated biphenyls and polychlorinated dibenzo-p-dioxins could require billions of dollars in the coming years. Harnessing microorganisms such as Dehalococcoides spp. that detoxify these compounds via removal of halogens may lead to cost-effective biotechnological approaches for remediation. | Dehalococcoides ethenogenes Strain 195 Reductively Dechlorinates Diverse Chlorinated Aromatic Pollutants | Fennel et al., 2004 | Link |
| Aerobic biotransformation of the diaryl ethers 2,7-dichlorodibenzo-p-dioxin and 1,2,3,4-tetrachlorodibenzo-p-dioxin by the dibenzo-p-dioxin-utilizing strain Sphingomonas wittichii RW1, producing corresponding metabolites, was demonstrated for the first time. Our strain transformed 2,7-dichlorodibenzo-p-dioxin, yielding 4-chlorocatechol, and 1,2,3,4-tetrachlorodibenzo-p-dioxin, producing 3,4,5,6-tetrachlorocatechol and 2-methoxy-3,4,5,6-tetrachlorophenol; all of these compounds were unequivocally identified by mass spectrometry both before and after N,O-bis(trimethylsilyl)-trifluoroacetamide derivatization by comparison with authentic standards. Additional experiments showed that strain RW1 formed a second metabolite, 2-methoxy-3,4,5,6-tetrachlorophenol, from the original degradation product, 3,4,5,6-tetrachlorocatechol, by methylation of one of the two hydroxy substituents. | Biotransformation of 2,7-Dichloro- and 1,2,3,4-Tetrachlorodibenzo-p-Dioxin by Sphingomonas wittichii RW1 | Hong et al., 2002 | Link |
| Polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs), commonly known as dioxins (PCDD/Fs), are toxic environmental pollutants formed from various sources. Elimination of these pollutants from the environment is a difficult task due to their persistent and ubiquitous nature. Removal of dioxins by biological degradation (biodegradation) is considered a feasible method as an alternative to other expensive physicochemical approaches. Biodegradation of dioxins has been extensively studied in several microorganisms, and details concerning biodiversity, biodegradation, biochemistry and molecular biology of this process have accumulated during the last three decades. There are several microbial mechanisms responsible for biodegradation of dioxins, including oxidative degradation by dioxygenase-containing aerobic bacteria, bacterial and fungal cytochrome P-450, fungal lignolytic enzymes, reductive dechlorination by anaerobic bacteria, and direct ether ring cleavage by fungi containing etherase-like enzymes. Many attempts have been made to bioremediate PCDD/Fs using this basic knowledge of microbial dioxin degradation. This review emphasizes the present knowledge and recent advancements in the microbial biotransformation, biodegradation and bioremediation of dioxins. | Recent developments in microbial biotransformation and biodegradation of dioxins | Chang et al., 2008 | Link |
| This study was aimed at finding microorganisms capable of biodegrading dioxins efficiently to develop a biotechnological treatment for decomposition of dioxins. Dibenzofuran (DBF) was used as a test substance to cultivate and to screen microbes active in degrading dioxins. Two microbial species (Pseudomonas aeruginosa and Xanthomonas maltophilia) which could degrade DBF were isolated from the sludge of a Kraft pulp mill wastewater treatment plant. The optimal conditions for biodegrading DBF were 30 and pH 7. Ps. aeruginosa, which had a high growth rate with DBF as carbon source showed high growth rates in the presence of such dioxins as dibenzo-p-dioxin, medium growth with 1-chlorodibenzo-p-dioxin, 2-chlorodibenzo-p-dioxin and 2,8-dichlorodibenzofuran, low growth with 2,6-dichlorodibenzo-p-dioxin and 1,2,3,4-tetrachlorodibenzo-p-dioxin and no growth with octachlorodibenzofuran. These results suggested that the growth rate of Ps. aeruginosa using dioxins as carbon source decreased with the increase in the degree of chlorine substitution. Activity of Ps. aeruginosa biodegrading dioxins was high for DBF, dibenzo-p-dioxin and 1-chlorodibenzo-p-dioxin and low for 2,8-dichlorodibenzofuran, 3,6-dichlorodibenzofuran, 2-chlorodibenzo-p-dioxin and 2,6-dichlorodibenzo-p-dioxin. These results suggested that there is a correlation between growth and the ability to biodegrade dioxins. On the other hand, the efficiency in the biodegradation of 1,2,3,4-tetrachlorodibenzo-p-dioxin was comparatively high although the growth rate on this dioxin was low. Hydroxydibenzofuran, 2-hydroxy-3-allyl-benzofuran and 2-carboxyvinyloxy phenyl acetic acid were identified by gas chromatograph-mass spectrometry (GC/MS) as products of DBF biodegradation by Ps. aeruginosa, suggesting a possible biodegradation pathway for dioxins. | Biodegradation of Dibenzofuran and Dioxins by Pseudomonas Aeruginosa and Xanthomonas Maltophilia | Ishiguro et al., 2010 | Link |
| Waste generation tends to surge in quantum as the population and living conditions grow. A group of structurally related chemicals of dibenzofurans and dibenzo-p-dioxins including their chlorinated congeners collectively known as dioxins are among the most lethal environmental pollutants formed during different anthropogenic activities. Removal of dioxins from the environment is challenging due to their persistence, recalcitrance to biodegradation, and prevalent nature. Dioxin elimination through the biological approach is considered both economically and environmentally as a better substitute to physicochemical conventional approaches. Bacterial aerobic degradation of these compounds is through two major catabolic routes: lateral and angular dioxygenation pathways. Information on the diversity of bacteria with aerobic dioxin degradation capability has accumulated over the years and efforts have been made to harness this fundamental knowledge to cleanup dioxin-polluted soils. This paper covers the previous decades and recent developments on bacterial diversity and aerobic bacterial transformation, degradation, and bioremediation of dioxins in contaminated systems. | Aerobic bacterial transformation and biodegradation of dioxins: a review | Saibu et al., 2020 | Link |
| The capability of anaerobic bacterial consortia from different environmental sources including soils, sewage sludges, and sediment of the river Saale (Germany) to dehalogenate chlorinated dioxins was compared using 1,2,3,4-tetrachlorodibenzo-p-dioxin (1,2,3,4-TCDD) as the model compound. The inocula were amended with mineral medium and organic acids and spiked with a high concentration (50 ?M) of 1,2,3,4-TCDD to stimulate microbial dehalogenating activity. Reductive dechlorination was observed to 1,3-dichlorodibenzo-p-dioxin (1,3-DCDD) as the main product and to minor amounts of the 1,2,4- and 1,2,3-trichlorodibenzo-p-dioxins (TrCDD) using incubations with Saale River sediment. No reaction was observed in the controls and in incubations with soils or sewage sludges. The dechlorination of 1,2,4- and 1,2,3-TrCDD was analyzed in separate subcultures. Reductive dechlorination of 1,2,4-TrCDD was a relative fast process (about 6 ?M converted within 58 days) and yielded only one product (1,3-DCDD). 1,2,3-TrCDD was slowly dechlorinated to equal amounts of 1,3- and 2,3-DCDD. These observations suggest that the main dechlorination route of 1,2,3,4-TCDD to 1,3-DCDD proceeds primarily via the removal of a lateral chlorine atom with 1,2,4-TrCDD as the intermediate. | Reductive Dechlorination of 1,2,3,4-Tetrachlorodibenzo-p-dioxin and Its Products by Anaerobic Mixed Cultures from Saale River Sediment | Ballerstedt et al., 1997 | Link |
| Polychlorinated dibenzo-p-dioxins (PCDDs) are toxic and widespread persistent organic pollutants (POPs). Cost-effective technologies for destroying or detoxifying PCDDs are in high demand. The overall purpose of this study was to develop a zero-valent zinc based technology for transforming toxic PCDDs to less- or non-toxic forms. We measured the dechlorination rates of 1,2,3,4-tetrachlorodibenzo-p-dioxin (1,2,3,4-TCDD) in the presence of zero-valent zinc under aqueous conditions, identified the daughter compounds of the reaction, and constructed possible pathways for the reactions. The reaction rates of daughter compounds with zero-valent zinc were also measured independently. Our results showed that the zero-valent zinc is a suitable candidate for reducing PCDDs. Reductive dechlorination of 1,2,3,4-TCDD was stepwise and complete to dibenzo-p-dioxin (DD) mainly via 1,2,4-trichlorodibenzo-p-dioxin (1,2,4-TrCDD), 1,3-dichlorodibenzo-p-dioxin (1,3-DCDD), 1-chlorodibenzo-p-dioxin (1-MCDD) to DD and via 1,2,4-TrCDD, 2,3-dichlorodibenzo-p-dioxin (2,3-DCDD), 2-chlorodibenzo-p-dioxin (2-MCDD) to DD. In each separate system, the observed half-lives of 1,2,3,4-TCDD, 1,2,3-TrCDD, 1,2,4-TrCDD, 1,2-DCDD, 1,3-DCDD, 1,4-DCDD and 2,3-DCDD are 0.56, 2.62, 5.71, 24.93, 41.53, 93.67 and 169.06 h respectively. The tendency of rate constant follows TCDD > TrCDD > DCDD. Our results suggest that zero-valent zinc is a suitable candidate for rapidly reducing highly chlorinated PCDDs to less or non-chlorinated daughter products. | Kinetics of reductive dechlorination of 1,2,3,4-TCDD in the presence of zero-valent zinc | Wang et al., 2008 | Link |
