Compound degraded:dibenzofuran(DF)
General Description (About POP compound)
Dibenzofuran is a heterocyclic organic compound with the chemical structure shown at right. It is an aromatic compound that has two benzene rings fused to a central furan ring. A family containing 135 individual, colorless compounds known as congeners with varying harmful health and environmental effects. They are produced as unwanted compounds during the manufacture of several chemicals and consumer products such as wood treatment chemicals, some metals, and paper products; also produced from the burning of municipal and industrial waste in incinerators, from exhaust of leaded gasoline, heat, or production of electricity. They are hazardous to the respiratory system, gastrointestinal system, liver, musculoskeletal system, skin and nervous system; and are toxic by inhalation, ingestion, and contact. Symptoms of exposure include frequent coughing, severe respiratory infections, chronic bronchitis, abdominal pain, muscle pain, acne rashes, skin color changes, unexpected weight loss, nonmalignant or malignant liver disease.
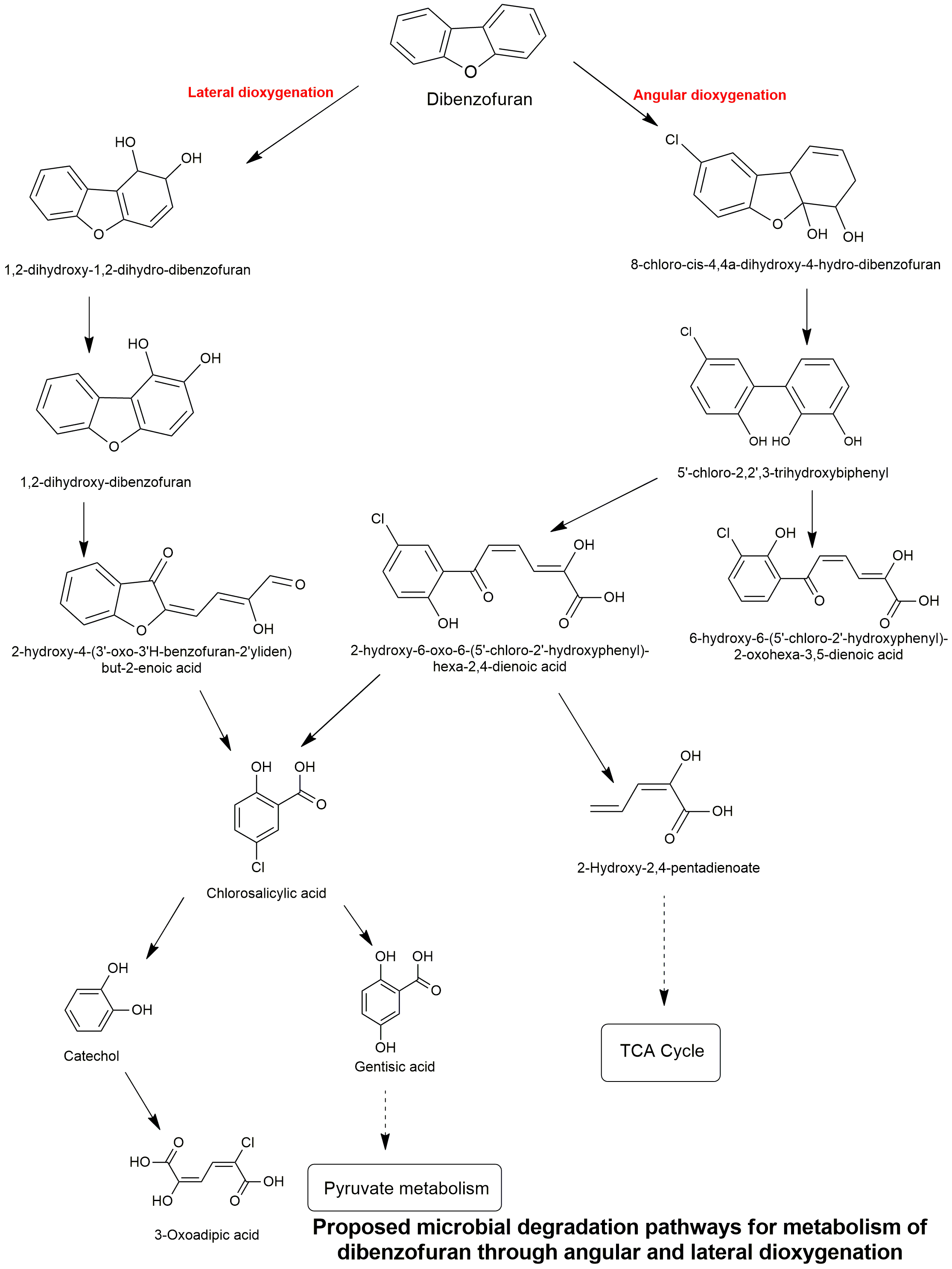
Biodegradation pathway
Publications
| Abstract | Title | Authors | Article Link |
|---|---|---|---|
| The efficacy of inoculation of single pure bacterial cultures into complex microbiomes, for example, in order to achieve increased pollutant degradation rates in contaminated material (that is, bioaugmentation), has been frustrated by insufficient knowledge on the behaviour of the inoculated bacteria under the specific abiotic and biotic boundary conditions. Here we present a comprehensive analysis of genome-wide gene expression of the bacterium Sphingomonas wittichii RW1 in contaminated non-sterile sand, compared with regular suspended batch growth in liquid culture. RW1 is a well-known bacterium capable of mineralizing dibenzodioxins and dibenzofurans. We tested the reactions of the cells both during the immediate transition phase from liquid culture to sand with or without dibenzofuran, as well as during growth and stationary phase in sand. Cells during transition show stationary phase characteristics, evidence for stress and for nutrient scavenging, and adjust their primary metabolism if they were not precultured on the same contaminant as found in the soil. Cells growing and surviving in sand degrade dibenzofuran but display a very different transcriptome signature as in liquid or in liquid culture exposed to chemicals inducing drought stress, and we obtain evidence for numerous ‘soil-specific’ expressed genes. Studies focusing on inoculation efficacy should test behaviour under conditions as closely as possible mimicking the intended microbiome conditions. | Genome-wide analysis of Sphingomonas wittichii RW1 behaviour during inoculation and growth in contaminated sand | Moreno-Forero and van der Meer. 2015 | Link |
| Sphingomonas wittichii strain RW1 (RW1) is one of the few strains that can grow on dibenzo-p-dioxin (DD). We conducted a transcriptomic study of RW1 using RNA-Seq to outline transcriptional responses to DD, dibenzofuran (DF), and the smectite clay mineral saponite with succinate as carbon source. The ability to grow on DD is rare compared to growth on the chemically similar DF even though the same initial dioxygenase may be involved in oxidation of both substrates. Therefore, we hypothesized the reason for this lies beyond catabolic pathways and may concern genes involved in processes for cell-substrate interactions such as substrate recognition, transport, and detoxification. Compared to succinate (SUC) as control carbon source, DF caused over 240 protein-coding genes to be differentially expressed, whereas more than 300 were differentially expressed with DD. Stress response genes were up-regulated in response to both DD and DF. This effect was stronger with DD than DF, suggesting a higher toxicity of DD compared to DF. Both DD and DF caused changes in expression of genes involved in active cross-membrane transport such as TonB-dependent receptor proteins, but the patterns of change differed between the two substrates. Multiple transcription factor genes also displayed expression patterns distinct to DD and DF growth. DD and DF induced the catechol ortho- and the salicylate/gentisate pathways, respectively. Both DD and DF induced the shared down-stream aliphatic intermediate compound pathway. Clay caused category-wide down-regulation of genes for cell motility and chemotaxis, particularly those involved in the synthesis, assembly and functioning of flagella. This is an environmentally important finding because clay is a major component of soil microbes’ microenvironment influencing local chemistry and may serve as a geosorbent for toxic pollutants. Similar to clay, DD and DF also affected expression of genes involved in motility and chemotaxis. | Sphingomonas wittichii Strain RW1 Genome-Wide Gene Expression Shifts in Response to Dioxins and Clay | Chai et al., 2016 | Link |
| Bacterial diversity and aerobic catabolic competence of dioxin-degrading bacterial strains isolated from a polluted soil in the tropics were explored. Isolation of bacteria occurred after 12 months of consecutive enrichment, with dioxin congeners serving as the only sources of carbon and energy. Seventeen strains that were isolated were subsequently screened for dioxin metabolic competence. Among these isolates, five had unique amplified ribosomal DNA restriction analysis (ARDRA) patterns out of which two exhibiting good metabolic competence were selected for further investigation. The two strains were identified as Bacillus sp. SS2 and Serratia sp. SSA1, based on their 16S rRNA gene sequences. Bacterial growth co-occurred with dioxin disappearance and near stoichiometric release of chloride for one ring of the chlorinated congeners. The overall percentage removal of dibenzofuran (DF) by strain SS2 was 93.87%; while corresponding values for 2,8-dichlorodibenzofuran (2,8-diCDF) and 2,7-dichlorodibenzo-p-dioxin (2,7-diCDD) were 86.22% and 82.30% respectively. In the case of strain SSA1, percentage removal for DF, 2,8-diCDF and 2,7-diCDD were respectively 98.9%, 80.97% and 70.80%. The presence of two dioxin dioxygenase catabolic genes (dxnA1 and dbfA1) was investigated. Only the dbfA1 gene could be amplified in SS2 strain. Results further revealed that strain SS2 presented higher expression levels for the alpha-subunit of DF dioxygenase (dbfA1) gene during growth with dioxins. The expression level for dbfA1 gene was higher when growing on DF than on the other chlorinated analogs. This study gives an insight into dioxin degradation, with the catabolic potential of strains SS2 and SSA1 (an enteric bacterium) within the sub-Sahara Africa. It further shows that dioxin catabolic potential might be more prevalent in different groups of microorganisms than previously believed. Few reports have demonstrated the degradation of chlorinated congeners of dioxins, particularly from sub-Saharan African contaminated systems. | Aerobic degradation of dichlorinated dibenzo-p-dioxin and dichlorinated dibenzofuran by bacteria strains obtained from tropical contaminated soil | Saibu et al., 2020 | Link |
| The dibenzofuran (DF)-degrading bacterium, Janibacter terrae strain XJ-1, was isolated from sediment from East Lake in Wuhan, China. This strain grows aerobically on DF as the sole source of carbon and energy; it has a doubling time of 12 hours at 30°C; and it almost completely degraded 100 mg/L?1 DF in 5 days, producing 2,2?,3-trihydroxybiphenyl, salicylic acid, gentisic acid, and other metabolites. The dbdA (DF dioxygenase) gene cluster in the strain is almost identical to that on a large plasmid in Terrabacter sp. YK3. Unlike Janibacter sp. strain YY-1, XJ-1 accumulates gentisic acid rather than catechol as a final product of DF degradation. | Biodegradation of Dibenzofuran by Janibacter terrae Strain XJ-1 | Jin et al., 2006 | Link |
| A moderate thermophilic dibenzofuran (DF) degrader, strain 4B1, was isolated from dioxin-contaminated soil in Vietnam under thermophilic condition. A 16S rRNA gene sequence analysis assigned the strain to genus Paenibacillus. The optimum growth temperature of strain 4B1 was 45°C with a doubling time of 2.7 h in the presence of DF as a sole carbon and energy source. The rate of its growth and DF-degradation were approximately 3-fold higher than those of a reference Paenibacillus sp. strain. The 4B1 strain degraded 89% of 1000 mg L?1 DF within 48 h cultivation at the optimum temperature. TBLASTN analysis based on its draft genome sequence revealed that this strain possessed a dbf gene cluster. The open reading frames (dbfA1A2RBC) in the cluster shared 99–100% identity with those of Paenibacillus sp. YK5, indicating that DF was likely degraded by an angular dioxygenation pathway in strain 4B1. Four genes in the dbf gene cluster (dbfA1A2BC) were partially induced by DF, which was observed by semi-quantitative RT-PCR. Quantitative PCR analysis of dbfA1 transcripts, encoding the alpha subunit of DF dioxygenase, indicated that dbfA1 was expressed 4-times higher than that of strain YK5 at 45°C. These results suggest that the faster growth and degradation of DF in strain 4B1 could be due to differences in transcriptional regulation of dbf cluster genes. | Isolation and characterization of a moderate thermophilic Paenibacillus naphthalenovorans strain 4B1 capable of degrading dibenzofuran from dioxin-contaminated soil in Vietnam | Thanh et al., 2019 | Link |
| A key enzyme in the degradation pathways of dibenzo-p-dioxin and dibenzofuran, namely, 2,2',3-trihydroxybiphenyl dioxygenase, which is responsible for meta cleavage of the first aromatic ring, has been genetically and biochemically analyzed. The dbfB gene of this enzyme has been cloned from a cosmid library of the dibenzo-p-dioxin- and dibenzofuran-degrading bacterium Sphingomonas sp. strain RW1 (R. M. Wittich, H. Wilkes, V. Sinnwell, W. Francke, and P. Fortnagel, Appl. Environ. Microbiol. 58:1005-1010, 1992) and sequenced. The amino acid sequence of this enzyme is typical of those of extradiol dioxygenases. This enzyme, which is extremely oxygen labile, was purified anaerobically to apparent homogeneity from an Escherichia coli strain that had been engineered to hyperexpress dbfB. Unlike most extradiol dioxygenases, which have an oligomeric quaternary structure, the 2,2',3-trihydroxybiphenyl dioxygenase is a monomeric protein. Kinetic measurements with the purified enzyme produced similar Km values for 2,2',3-trihydroxybiphenyl and 2,3-dihydroxybiphenyl, and both of these compounds exhibited strong substrate inhibition. 2,2',3-Trihydroxydiphenyl ether, catechol, 3-methylcatechol, and 4-methylcatechol were oxidized less efficiently and 3,4-dihydroxybiphenyl was oxidized considerably less efficiently. | Characterization of 2,2',3-trihydroxybiphenyl dioxygenase, an extradiol dioxygenase from the dibenzofuran- and dibenzo-p-dioxin-degrading bacterium Sphingomonas sp. strain RW1 | Happe et al., 1993 | Link |
| Sphingomonas wittichii RW1 is one of a few strains known to grow on the related compounds dibenzofuran (DBF) and dibenzo-p-dioxin (DXN) as the sole source of carbon. Previous work by others (B. Happe, L. D. Eltis, H. Poth, R. Hedderich, and K. N. Timmis, J Bacteriol 175:7313–7320, 1993, https://doi.org/10.1128/jb.175.22.7313-7320.1993) showed that purified DbfB had significant ring cleavage activity against the DBF metabolite trihydroxybiphenyl but little activity against the DXN metabolite trihydroxybiphenylether. We took a physiological approach to positively identify ring cleavage enzymes involved in the DBF and DXN pathways. Knockout of dbfB on the RW1 megaplasmid pSWIT02 results in a strain that grows slowly on DBF but normally on DXN, confirming that DbfB is not involved in DXN degradation. Knockout of SWIT3046 on the RW1 chromosome results in a strain that grows normally on DBF but that does not grow on DXN, demonstrating that SWIT3046 is required for DXN degradation. A double-knockout strain does not grow on either DBF or DXN, demonstrating that these are the only ring cleavage enzymes involved in RW1 DBF and DXN degradation. The replacement of dbfB by SWIT3046 results in a strain that grows normally (equal to the wild type) on both DBF and DXN, showing that promoter strength is important for SWIT3046 to take the place of DbfB in DBF degradation. Thus, both dbfB- and SWIT3046-encoded enzymes are involved in DBF degradation, but only the SWIT3046-encoded enzyme is involved in DXN degradation. | Separate Upper Pathway Ring Cleavage Dioxygenases Are Required for Growth of Sphingomonas wittichii Strain RW1 on Dibenzofuran and Dibenzo- p-Dioxin | Mutter and Zylstra. 2021 | Link |
| Sphingomonas wittichii RW1 grows on the two related compounds dibenzofuran (DBF) and dibenzo-p-dioxin (DXN) as the sole source of carbon. Previous work by others (P. V. Bunz, R. Falchetto, and A. M. Cook, Biodegradation 4:171-178, 1993, https://doi/org/10.1007/BF00695119) identified two upper pathway meta cleavage product hydrolases (DxnB1 and DxnB2) active on the DBF upper pathway metabolite 2-hydroxy-6-oxo-6-(2-hydroxyphenyl)-hexa-2,4-dienoate. We took a physiological approach to determine the role of these two enzymes in the degradation of DBF and DXN by RW1. Single knockouts of either plasmid-located dxnB1 or chromosome-located dxnB2 had no effect on RW1 growth on either DBF or DXN. However, a double-knockout strain lost the ability to grow on DBF but still grew normally on DXN, demonstrating that DxnB1 and DxnB2 are the only hydrolases involved in the DBF upper pathway. Using a transcriptomics-guided approach, we identified a constitutively expressed third hydrolase encoded by the chromosomally located SWIT0910 gene. Knockout of SWIT0910 resulted in a strain that no longer grows on DXN but still grows normally on DBF. Thus, the DxnB1 and DxnB2 hydrolases function in the DBF but not the DXN catabolic pathway, and the SWIT0190 hydrolase functions in the DXN but not the DBF catabolic pathway. IMPORTANCE S. wittichii RW1 is one of only a few strains known to grow on DXN as the sole source of carbon. Much of the work deciphering the related RW1 DXN and DBF catabolic pathways has involved genome gazing, transcriptomics, proteomics, heterologous expression, and enzyme purification and characterization. Very little research has utilized physiological techniques to precisely dissect the genes and enzymes involved in DBF and DXN degradation. Previous work by others identified and extensively characterized two RW1 upper pathway hydrolases. Our present work demonstrates that these two enzymes are involved in DBF but not DXN degradation. In addition, our work identified a third constitutively expressed hydrolase that is involved in DXN but not DBF degradation. Combined with our previous work (T. Y. Mutter and G. J. Zylstra, Appl Environ Microbiol 87:e02464-20, 2021, https://doi.org/10.1128/AEM.02464-20), this means that the RW1 DXN upper pathway involves genes from three very different locations in the genome, including an initial plasmid-encoded dioxygenase and a ring cleavage enzyme and hydrolase encoded on opposite sides of the chromosome. | Differential Roles of Three Different Upper Pathway meta Ring Cleavage Product Hydrolases in the Degradation of Dibenzo- p-Dioxin and Dibenzofuran by Sphingomonas wittichii Strain RW1 | Mutter and Zylstra. 2021 | Link |
| Sphingomonas wittichii RW1 is a bacterium of interest due to its ability to degrade polychlorinated dioxins, which represent priority pollutants in the USA and worldwide. Although its genome has been fully sequenced, many questions exist regarding changes in protein expression of S. wittichii RW1 in response to dioxin metabolism. We used difference gel electrophoresis (DIGE) and matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) to identify proteomic changes induced by growth on dibenzofuran, a surrogate for dioxin, as compared to acetate. Approximately 10% of the entire putative proteome of RW1 could be observed. Several components of the dioxin and dibenzofuran degradation pathway were shown to be upregulated, thereby highlighting the utility of using proteomic analyses for studying bioremediation agents. This is the first global protein analysis of a microorganism capable of utilizing the carbon backbone of both polychlorinated dioxins and dibenzofurans as the sole source for carbon and energy. | Proteomic Profiling of the Dioxin-Degrading Bacterium Sphingomonas wittichii RW1 | Colquhoun et al., 2012 | Link |
| Anaerobic enrichment cultures derived from contaminated Kymijoki River sediments dechlorinated 1,2,3,4-tetrachlorodibenzofuran (1,2,3,4-tetra-CDF), octachlorodibenzofuran (octa-CDF) and 1,2,3,4-tetrachlorodibenzo-p-dioxin (1,2,3,4-tetra-CDD). 1,2,3,4-tetra-CDF was dechlorinated via 1,2,3-, 2,3,4-, and 1,3,4/1,2,4-tri-CDFs to 1,3-, 2,3-, and 2,4-di-CDFs and finally to 4-mono-CDF. The dechlorination rate of 1,2,3,4-tetra-CDF was generally slower than that of 1,2,3,4-tetra-CDD. The rate and extent of 1,2,3,4-tetra-CDD dechlorination was enhanced by addition of pentachloronitrobenzene (PCNB) as a co-substrate. Dechlorination of spiked octa-CDF was observed with the production of hepta-, hexa-, penta- and tetra-CDFs over 6 months. Two major phylotypes of the Chloroflexi community showed an increase, one of which was identical to the Dehalococcoides mccartyi Pinellas subgroup. A set of twelve putative reductive dehalogenase (rdh) genes increased in abundance with addition of 1,2,3,4-tetra-CDF, 1,2,3,4-tetra-CDD and/or PCNB. This information will aid in understanding how indigenous microbial communities impact the fate of PCDFs and in developing strategies for bioremediation of PCDD/F contaminated sediments. | Enriching for microbial reductive dechlorination of polychlorinated dibenzo-p-dioxins and dibenzofurans | Liu et al., 2014 | Link |
