Compound degraded:Dicofol
General Description (About POP compound)
Dicofol is an organochlorine miticide used on a wide variety of fruit, vegetable, ornamental and field crops. DDT is one of the intermediates used in the production of dicofol. In 1986, use of dicofol was temporarily canceled by the EPA because of concerns raised by high levels of DDT contamination. However, it was reinstated when it was shown that modern manufacturing processes can produce technical grade dicofol which contains less than 0.1% DDT
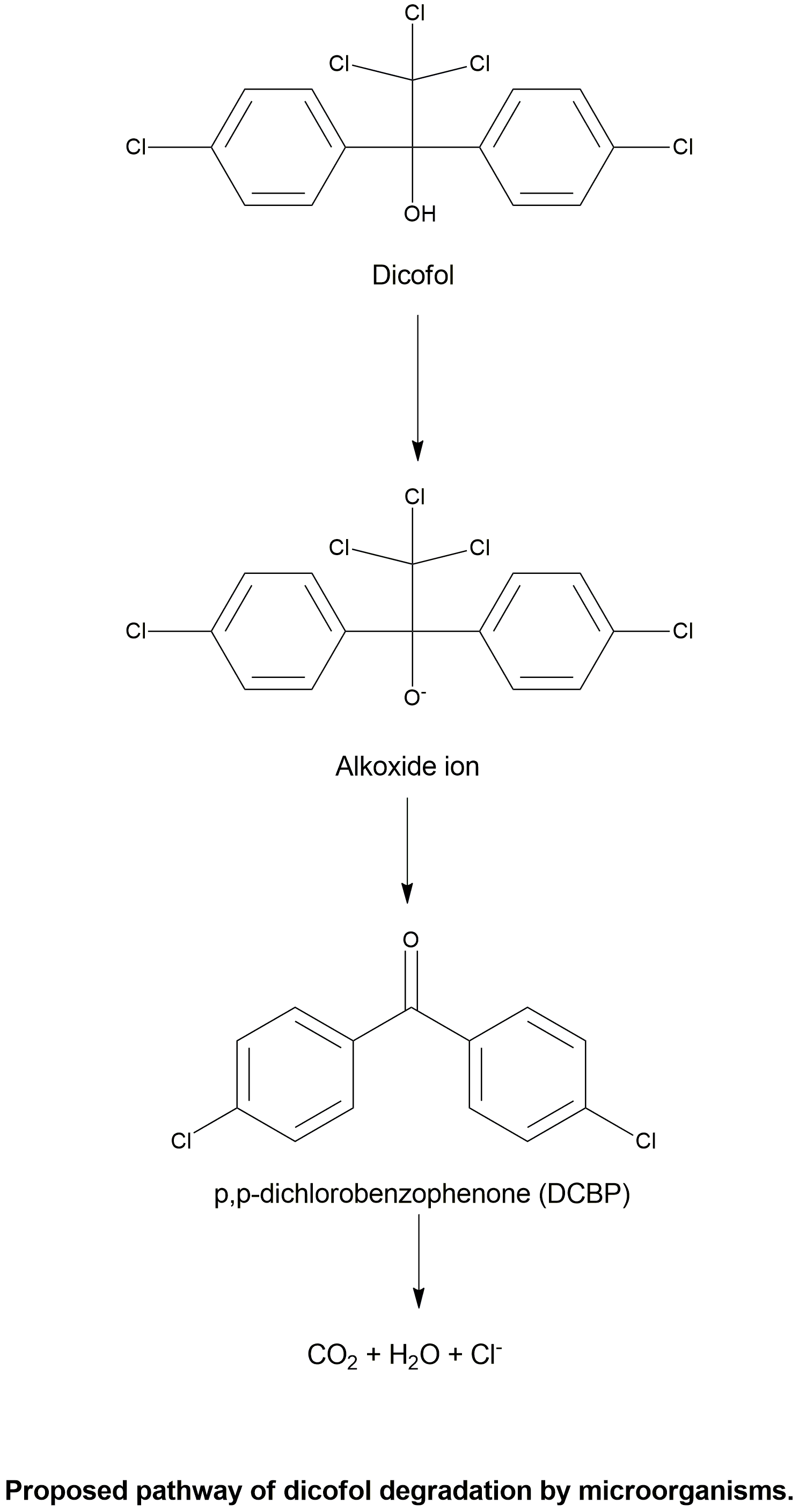
Biodegradation pathway
Publications
| Abstract | Title | Authors | Article Link |
|---|---|---|---|
| Dicofol is an organochlorine insecticide widely used to prevent pests worldwide. Consequently, serious environmental problems have arisen from the application of dicofol. Bioremediation is an effective solution for dicofol persistence in the environment. In this study, a bacterial strain D-2, identified to genus Microbacterium, capable of degrading dicofol was isolated from dicofol-contaminated agricultural soil. This represents the first dicofol degrading bacterium isolated from this genus. Microbacterium sp. D-2 degraded 50 mg/L dicofol within 24 h at a rate of 85.1%. Dicofol was dechlorinated by D-2 and the further degradation metabolite was indentified as p,p?-dichlorobenzophenone(DCBP). Soils inoculated with Microbacterium sp. D-2 degraded 81.9% of the dicofol, while soils without D-2 only degraded 20.5% of the dicofol present. This finding suggests that strain D-2 has great potential in bioremediation of dicofol-contaminated soils. | Biodegradation of dicofol by Microbacterium sp. D-2 isolated from pesticide-contaminated agricultural soil | Lu et al., 2019 | Link |
| Organic micropollutants are often found in domestic and industrial effluents. Thus, it is important to learn their fate, the metabolites generated and their sorption during biological treatment processes. This work investigated the biodegradation of 14C-dicofol organochloride during wastewater aerobic treatment and sludge anaerobic biodigestion. The performance of these processes was evaluated by physical–chemical parameters. Radioactivity levels were monitored in both treatments, and residues of dicofol (DCF) and dichlorobenzophenone (DBP) were quantified by HPLC/UV. The efficiency of the aerobic and anaerobic processes was slightly reduced in the presence of DCF and DBP. After aerobic treatment, only 0.1% of DCF was mineralized, and 57% of radioactivity remained sorbed on biological sludge as DBP. After 18 days of anaerobiosis, only 3% of DCF and 5% of DBP were detected in the sludge. However, 70% of radioactivity remained in the sludge, probably as other metabolites. Dicofol was biodegraded in the investigated process, but not mineralized. | Biodegradation of 14C-dicofol in wastewater aerobic treatment and sludge anaerobic biodigestion | da M Oliveira et al., 2012 | Link |
| In the present investigation, the degradation of the acaricide dicofol (also known as kelthane) was investigated with special emphasis on generation of p,p?-dichlorobenzophenone (DCBP) under alkaline conditions as well as induced by UV-light. Dicofol was also incubated in the presence and absence of microsomal preparations to measure potential metabolic formation of DCBP. The results indicate that the degradation of dicofol to DCBP primarily proceeds as an abiotic process via hydroxide ion catalysed elimination of a trichloromethyl anion. The generated anion picks up a proton from the solvent to generate chloroform. Microsomal metabolism does not appear to play a major role in the degradation of dicofol. | Dicofol degradation to p,p?-dichlorobenzophenone – A potential antiandrogen | Thiel et al., 2011 | Link |
| From the series of organochlorine insecticides, kelthane and methoxychlor were most easily degraded by the bacteria tested. The amount of metabolites from methoxychlor was low and recovery of the insecticide from a bacterial suspension was not possible. Details for biodegradation of methoxychlor are described. Anaerobiosis, neutral pH, and a large number of bacteria are necessary. Some reducing substances, however, may replace anaerobic conditions. The contact of bacteria with kelthane or methoxychlor liberates Cl? but the quantity does not correspond to the expected theoretical value. A dechlorination of the ethane portion of the molecule is hypothesized. | Biodegradation of methoxychlor and kelthane | Van Duck and van de Voorde. 1976 | Link |
