Compound degraded:Dieldrin
General Description (About POP compound)
Dieldrin (a metabolite of aldrin as well as a marketed pesticide) is a highly persistent organochlorine insecticide, it was used to control locusts and tropical disease vectors, such as Glossina spp. and mosquitoes. Former industrial uses include timber preservation, termite proofing of plastic and rubber coverings of electrical and telecommunications cables, of plywood and building boards, etc.
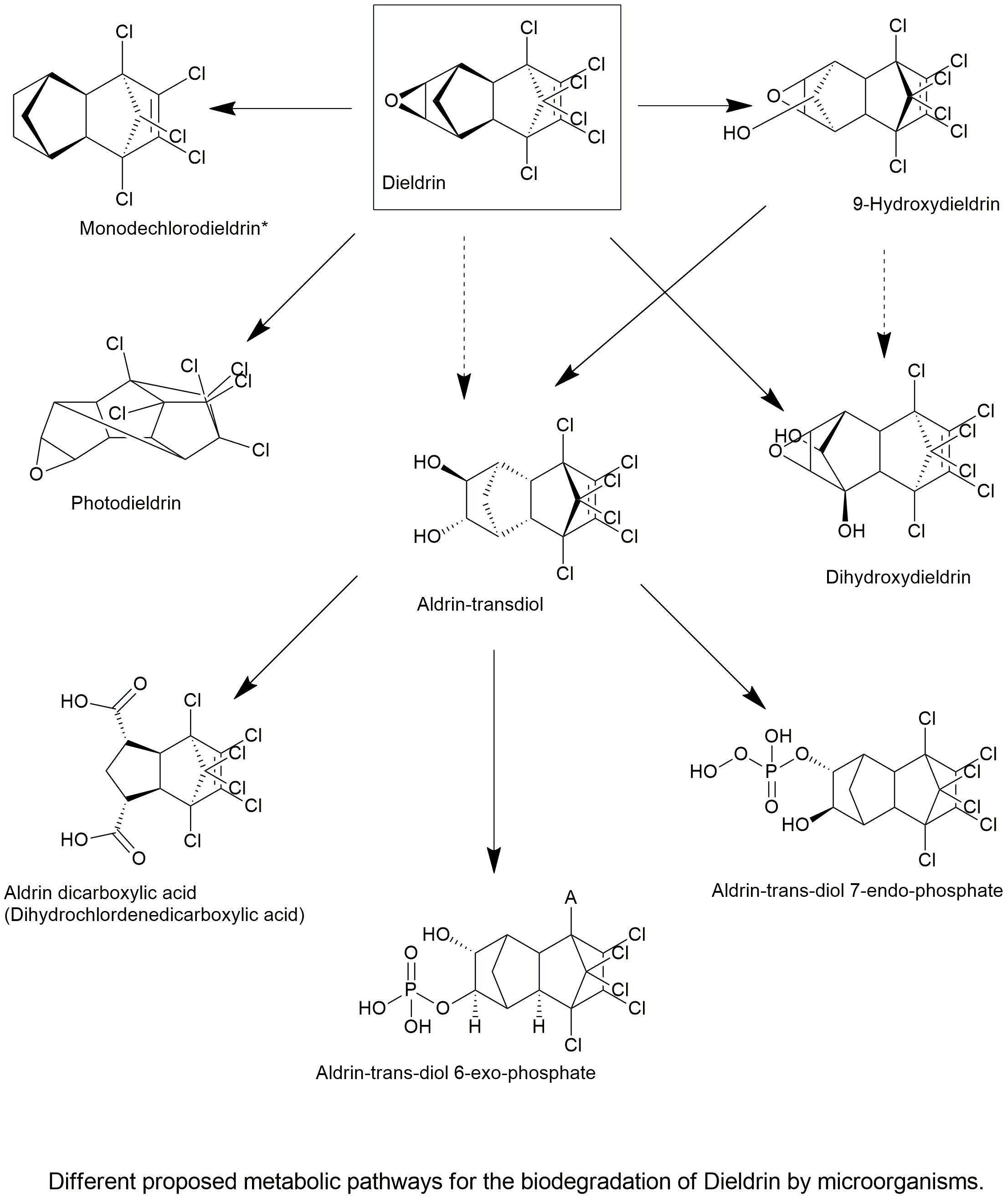
Biodegradation pathway
Publications
| Abstract | Title | Authors | Article Link |
|---|---|---|---|
| An aerobic dieldrin-degrading fungus, Mucor racemosus strain DDF, was isolated from a soil to which endosulfan had been annually applied for more than 10 years until 2008. Strain DDF degraded dieldrin to 1.01 ?M from 14.3 ?M during a 10-day incubation at 25 °C. Approximately 0.15 ?M (9%) of aldrin trans-diol was generated from the dieldrin degradation after a 1-day incubation. The degradation of dieldrin by strain DDF was detected over a broad range of pH and concentrations of glucose and nitrogen sources. Extracellular fluid without mycelia also degraded dieldrin. Strain DDF degraded not only dieldrin but also heptachlor, heptachlor epoxide, endosulfan, endosulfan sulfate, DDT, and DDE. Endosulfan sulfate and heptachlor were degraded by 0.64 ?M (95%) and 0.75 ?M (94%), respectively, whereas endosulfan and DDE were degraded by 2.42 ?M (80%) and 3.29 ?M (79%), respectively, and DDT and heptachlor epoxide were degraded by 6.95 ?M (49.3%) and 5.36 ?M (67.5%), respectively, compared with the control, which had a concentration of approximately 14 ?M. These results suggest that strain DDF could be a candidate for the bioremediation of sites contaminated with various persistent organochlorine pesticides including POPs. | Biodegradation of Dieldrin by a Soil Fungus Isolated from a Soil with Annual Endosulfan Applications | Kataoka. 2010 | Link |
| An aerobic dieldrin-degrading fungus, Mucor racemosus strain DDF, and two aerobic endosulfan-degrading fungal strains, Mortierella sp. strains W8 and Cm1-45, were isolated from soil contaminated with organochlorine pesticides. Strain DDF degraded more than 90% dieldrin during 10-days of incubation at 25°C and showed the production of a small amount of aldrin trans-diol. Moreover, strain DDF reduced levels of aldrin trans-diol while producing unknown metabolites that were determined to be aldrin trans-diol exo- and endo-phosphates. On the other hand, Mortierella sp. strains W8 and Cm1-45 degraded more than 70% and 50% of ? and ?-endosulfan, respectively, over 28 days at 25°C, in liquid cultures containing initial concentrations of 8.2?µM of each substance. Only a small amount of endosulfan sulfate, a persistent metabolite, was detected in the both cultures, while these strains could not degrade endosulfan sulfate when this compound was provided as the initial substrate. Both strains generate endosulfan diol as a first step in the degradation of endosulfan, then undergo further conversion to endosulfan lactone. | Biodegradability and biodegradation pathways of chlorinated cyclodiene insecticides by soil fungi | Kataoka. 2018 | Link |
| We isolated a novel aerobic dieldrin-degrading bacterium from an enrichment culture in a soil–charcoal perfusion system. Enrichment culture using a soil–charcoal perfusion system was an effective way to obtain microorganisms that degrade recalcitrant compounds. The soil–charcoal perfusion was performed using aldrin trans-diol, which was a metabolite of dieldrin. Aldrin trans-diol had higher bioavailability (2.5 mg/l) than dieldrin (0.1–0.25 mg/l), therefore it is possible for microorganisms to utilize it as a substrate in soil. After 100 days of circulation and three exchanges of the medium, the enriched charcoal was harvested and a bacterium isolated. The isolate was designated as strain KSF27 and was found to be closely related to Pseudonocardia spp. as determined by 16S rRNA sequencing analysis. Strain KSF27 degraded aldrin trans-diol by 0.05 ?mol/l from an initial concentration of 25.5 ?mol/l. The metabolite of aldrin trans-diol was detected by HPLC/MS and determined to be aldrindicarboxylic acid based on retention time and the MS fragment. Moreover, strain KSF27 degraded dieldrin from 14.06 ?mol/l to 2.01 ?mol/l over a 10-day incubation at 30 °C. This strain degraded dieldrin and other persistent organochlorine pesticides, such as ?-endosulfan, ?-endosulfan, endosulfan sulfate, heptachlor, heptachlor epoxide and chlordecone. | Isolation and identification of dieldrin-degrading Pseudonocardia sp. strain KSF27 using a soil–charcoal perfusion method with aldrin trans-diol as a structural analog of dieldrin | Sakakibara et al., 2011 | Link |
| Dieldrin is one of the most persistent organic pollutants, and its oxidative degradation pathways by aerobic microorganisms to 6,7-trans-dihydroxydihydroaldrin (otherwise known as aldrin-trans-diol) and 9-hydroxydieldrin are well documented. The dieldrin-degrading fungus, Mucor racemosus strain DDF, can decrease dieldrin levels with simultaneous production of a small amount of aldrin-trans-diol. A reduction in the levels of aldrin-trans-diol by strain DDF has also been observed. Based on these results, it has been suggested that strain DDF transforms dieldrin to more polar compounds via aldrin-trans-diol. We have conducted a study to identify the metabolites arising from aldrin-trans-diol. The results showed that strain DDF gave reduced levels of aldrin-trans-diol and also produced unknown metabolites. Ultra performance liquid chromatography-electrospray ionization-mass spectroscopy (UPLC-ESI-MS) analysis indicated the metabolites to be either sulfated- or phosphorylated- derivatives of aldrin-trans-diol, but with the metabolites retaining six chlorine atoms. Therefore, the candidate derivatives were synthesized and the retention times of the natural metabolite and the synthetic phosphate were compared. As a result of a co-injection experiment, the metabolites were determined to be aldrin-trans-diol exo- and endo-phosphates. These results were also supported by high-resolution-fast atom bombardment-mass spectrometry (HR-FAB-MS) of the natural metabolite (? = 0.63 ppm). Phosphorylation of aldrin-trans-diol is the first reported example of phosphate conjugation in microorganisms. | Novel phosphorylation of aldrin-trans-diol by dieldrin-degrading fungus Mucor racemosus strain DDF | Yamakazi et al., 2014 | Link |
| Twelve strains belonging to the genus Cordyceps were investigated for their ability to degrade organochlorine pesticide dieldrin. Based on the screening results, we further investigated Cordyceps militaris KS-92 and Cordyceps brongniartii ATCC66779 to determine their degradation capacity and metabolic products towards dieldrin. C. militaris KS-92 and C. brongniartii ATCC66779 removed about 45% and 36% of dieldrin in PDB medium, respectively, after 28 days of incubation. A hydrolysis product, 6,7-dihydroxydihydroaldrin, was detected as a initial metabolite of dieldrin in both fungal cultures using GC/MS analysis. C. militaris KS-92 particularly can degrade dieldrin to dihydrochlordenedicarboxylic acid through oxidation of 6,7-dihydroxydihydroaldrin or directly oxidation of dieldrin. The results suggested that dieldrin was metabolized to hydrophilic/low-toxicity products by selected fungi. | Biodegradation of Dieldrin by Cordyceps Fungi and Detection of Metabolites | Tang et al., 2013 | Link |
