Compound degraded:Heptachlor
General Description (About POP compound)
Heptachlor is a non-systemic insecticide with contact, stomach, and some respiratory action. It is used to control termites, ants and soil insects in cultivated and non-cultivated soils. Applied as a seed treatment, soil treatment, or directly to foliage. Also used for control of household insects
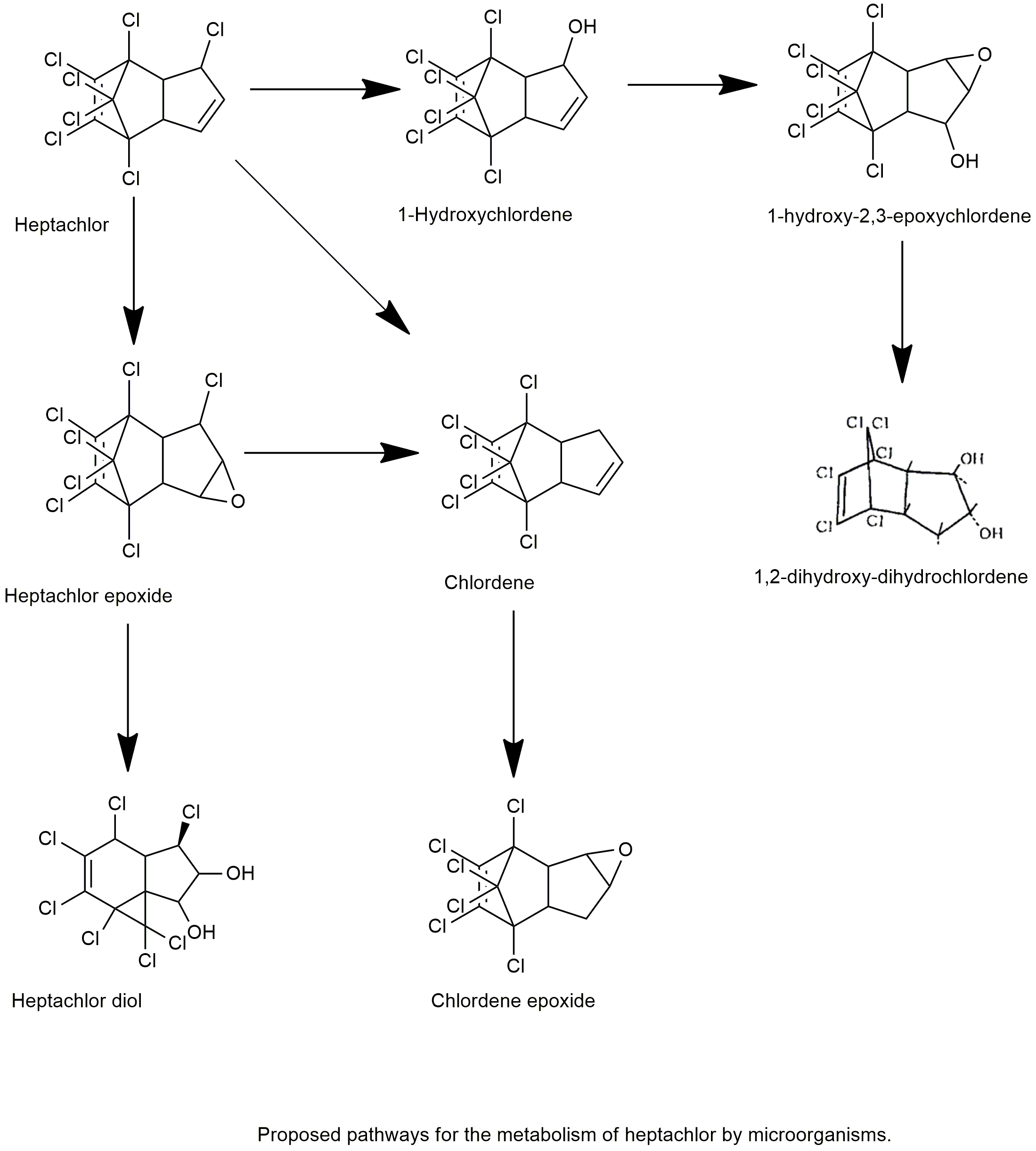
Biodegradation pathway
Publications
| Abstract | Title | Authors | Article Link |
|---|---|---|---|
| White rot fungi of the genus Phlebia have demonstrated a high capacity to degrade organic pollutants, including polychlorinated dibenzo-p-dioxins and polychlorinated biphenyls. In this study, we evaluated the ability of 18 white rot fungi species of genus Phlebia to degrade heptachlor and heptachlor epoxide, and described the metabolic pathways by selected white rot fungi. Phlebia tremellosa, Phlebia brevispora and Phlebia acanthocystis removed about 71%, 74% and 90% of heptachlor, respectively, after 14 days of incubation. A large amount of heptachlor epoxide and a small amount of 1-hydroxychlordene and 1-hydroxy-2,3-epoxychlordene were detected as metabolic products of heptachlor from most fungal cultures. The screening of heptachlor epoxide-degrading fungi revealed that several fungi are capable of degrading heptachlor epoxide, which is a recalcitrant metabolite of heptachlor. Phlebia acanthocystis, P. brevispora, Phlebia lindtneri and Phlebia aurea removed about 16%, 16%, 22% and 25% of heptachlor epoxide, respectively, after 14 days of incubation. Heptachlor diol and 1-hydroxy-2,3-epoxychlordene were produced in these fungal cultures as metabolites, suggesting that the hydrolysis and hydroxylation reaction occur in the epoxide ring and in position 1 of heptachlor epoxide, respectively. | Metabolism of organochlorine pesticide heptachlor and its metabolite heptachlor epoxide by white rot fungi, belonging to genus Phlebia | Xiao et al., 2011 | Link |
| The ability of certain white-rot fungi (WRF) inocula to transform heptachlor and heptachlor epoxide and its application in artificially contaminated soil were investigated. Fungal inoculum of Pleurotus ostreatus eliminated approximately 89 % of heptachlor after 28 days of incubation, and chlordene was detected as the primary metabolite. The fungal inoculum of Pleurotus ostreatus had the highest ability to degrade heptachlor epoxide; approximately 32 % were degraded after 28 days of incubation, and heptachlor diol was detected as the metabolite product. Because Pleurotus ostreatus transformed heptachlor into a less toxic metabolite and could also effectively degrade heptachlor epoxide, it was then selected to be applied to artificially contaminated soil. The spent mushroom waste (SMW) of Pleurotus ostreatus degraded heptachlor and heptachlor epoxide by approximately 91 and 26 %, respectively, over 28 days. This finding indicated that Pleurotus ostreatus SMW could be used to bioremediate heptachlor- and heptachlor epoxide-contaminated environments. | Biodegradation of heptachlor and heptachlor epoxide-contaminated soils by white-rot fungal inocula | Purnomo et al., 2014 | Link |
| [14C]Heptachlor is metabolized by the freshwater microcrustacean, Daphnia magna, to 1-hydroxychlordene, 1-ketochlordene, 1-hydroxy-2,3-epoxychlordene, and their glucosides, sulfates, and other conjugates. 1-Hydroxychlordene is converted to 1-ketochlordene, indicating that in vivo formation of 1-ketochlordene may proceed via oxidative dehydrochlorination of HC-Cl in heptachlor to CH?OH (1-hydroxychlordene) followed by oxidation of the latter to 1-ketochlordene. The ketone formation from heptachlor may not result via a chlorohydrin intermediate. | Oxidative dehydrochlorination of heptachlor by Daphnia magna | Feroxz et al., 1990 | Link |
| The metabolic fate oftrans-nonachlor and heptachlor in rats was studiedin vivo andin vitro by using microsomal preparations of the liver. During in vivo studies the rate of excretion into urine and feces was monitored. The major metabolic products were collected from the feces and their chemical structures were determined by using various chromatographic and spectroscopic techniques. The immediate major metabolic product oftrans-nonachlor istrans-chlordane which is further converted to 1,2-dichlorochlordene and to oxychlordane. From the latter metabolite, two major stable products, 1-hydroxy-2-chlorochlordene and 1-hydroxy-2-chloro-2,3-epoxy-chlordene are formed. Another route of metabolism is a direct formation of chlordene chlorohydrin which acts as a precursor for 1,2-trans-dihydroxydihydrochlordene. The major metabolic products of heptachlor were heptachlor epoxide, 1-exo-hydroxyheptachlor epoxide and 1,2-dihydroxy-dihydrochlordene. | Metabolism oftrans-nonachlor and related chlordane components in rat and man | Tashiro and Matsumura. 1978 | Link |
| Degradation of aldrin (1,2,3,4,10,10-Hexachloro-1,4,4a,5,8,8a-hexahydro-1,4:5-8-dimethanonaphthalene), heptachlor (1H-1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro-4,7-methano indene), dieldrin (1a?,2?,2a?,3?,6?,6a?,7?,7a?)-3,4,5,6,9,9-Hexachloro-1a,2,2a,3,6,6a,7,7a-octahydro-2,7:3,6-d-methanonaphtha[2,3-b]oxirene, and heptachlor epoxide (1a?, 1b?,2?,5?,5??,6?,6a?-2,3,4,5,6,7,7-Heptachloro-1a,1b,5,5a,6,6a-hexahydro-2,5-methano-2H-inden[1,2-b]-oxirene) was tested using free cultures of Pseudomonas fluorescens under controlled conditions. Pesticide concentrations were monitored by gas chromatography during 120 h. Percentages of degradation and biodegradation rates (BDR) were calculated. Data showed a trend suggesting a relation between chemical structure and degradability. Degradation kinetics for each pesticide tested showed that the highest degradation rates were found in the first 24 h. Kinetics data were adjusted to an empirical equation in order to predict their behavior, and the correlation coefficients obtained were satisfactory. Gas chromatography/mass spectrometry (GC/MS) analysis of the final extracts allowed the identification of chlordene and monodechlorodieldrin, which have been reported as final metabolite produced in the biodegradation of this kind of compounds. Regarding adsorption of pesticides on activated vegetal carbon, we concluded that removal efficiencies between 95.45 and 97.18% can be reached, depending on the pesticide and the carbon dose applied. The values for K from the Freundlich equation were quite similar for the four pesticides (between 1.0001 and 1.04), whereas the n values were quite different for each pesticide in the following order of affinity: dieldrin > aldrin > heptachlor epoxide > heptachlor. Equilibrium times, very important for scaling up the process, were between 43 min and 1 h, for the heptachlor epoxide and the heptachlor, respectively. | Heptachlor and Its Metabolite: Accumulation and Degradation in Sediment | Pokethitiyook and Poolpak. 2012 | Link |
