Compound degraded:Hexachlorobuta-1,3-diene
General Description (About POP compound)
Hexachlorobuta-1,3-diene is a chlorinated aliphatic diene with niche applications but is most commonly used as a solvent for other chlorine-containing compounds. It is primarily produced in chlorinolysis plants as a by-product in the production of carbon tetrachloride and tetrachloroethene. Chlorinolysis is a radical chain reaction that occurs when hydrocarbons are exposed to chlorine gas under pyrolytic conditions. The hydrocarbon is chlorinated and the resulting chlorocarbons are broken down. Hexachloro-1,3-butadiene is also used in some industrial processes, such as heat transfer liquid, reactant in chemical syntheses, organic solvent, wash liquor for hydrocarbon removal.
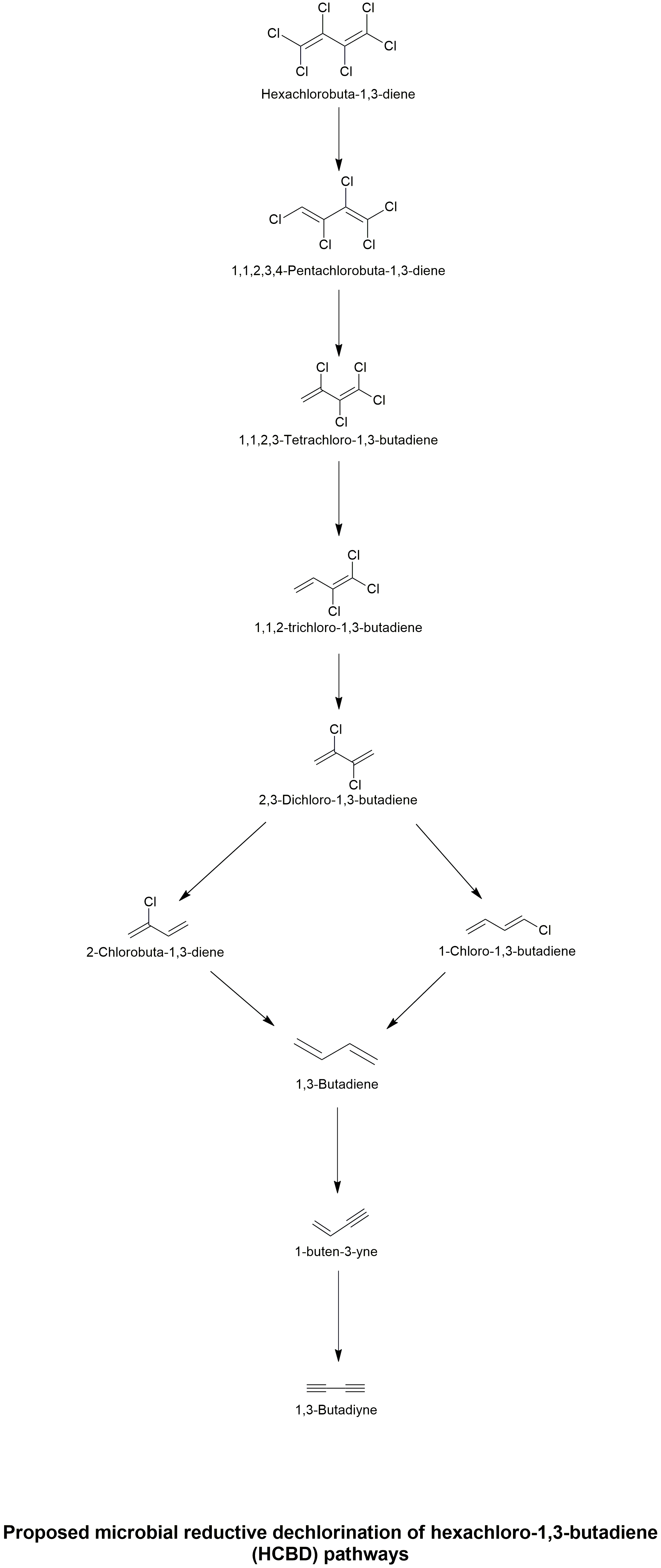
Biodegradation pathway
Publications
| Abstract | Title | Authors | Article Link |
|---|---|---|---|
| Sequential reductive dechlorination of hexachloro-1,3-butadiene (HCBD) was achieved by a mixed, methanogenic culture enriched from a contaminated estuarine sediment. Both methanol and lactate served as carbon and electron sources. Methanol was stoichiometrically converted to methane, whereas lactate was fermented to propionate and acetate and then to methane. Lactate and propionate fermentation, as well as methanogenesis were not inhibited at 0.4 mg HCBD/l, the normal enrichment culture HCBD feeding level. At a higher initial HCBD level of 1.5 mg HCBD/l, propionate fermentation and acetoclastic methanogenesis were inhibited while, after a lag time, enhanced HCBD dechlorination rates were observed. While lactate fermentation was not inhibited at high concentrations (>25 mM) of 2-bromoethanesulfonate (BES), both propionate fermentation and methanogenesis were completely inhibited, although the HCBD dechlorination rate was not affected. Therefore, methanogens were not likely responsible for the observed dechlorination of HCBD in the enrichment culture. The predominant HCBD dechlorination products were isomers of tri- and dichloro-1,3-butadiene. Traces of a monochloro-1,3-butadiene isomer were also detected. Although extensive dechlorination of HCBD was achieved by the enrichment culture, the detoxification efficiency of this process remains unclear because the potential inhibitory effect of the HCBD transformation products is unknown. | Microbial reductive dechlorination of hexachloro-1,3-butadiene in a methanogenic enrichment culture | Booker and Pavlostathis. 2000 | Link |
| Environments contaminated with mixtures of chlorinated hydrocarbons represent a formidable challenge for bioremediation because biodegradation of all components of the mixture must be demonstrated. In this study a soil site contaminated with hexachloro-1,3-butadiene (HCBD), hexachlorobenzene (HCB), and perchloroethene (PCE) was investigated. Environmental parameters (including toxicity) and microbial community composition were characterized. The lack of scientific literature on HCBD biodegradation led to attempts to develop HCBD-respiring enrichment cultures and to test the hypothesis that known PCE-degrading cultures could dechlorinate HCBD. No HCBD dechlorination was observed. An alternative approach, using electron shuttles to degrade the mixture of chlorinated hydrocarbons, was compared with the activity of zero-valent iron. The authors conclude that electron shuttles offer promise for the in situ treatment of mixtures of chlorinated hydrocarbons. | Comparison of Reductive Dechlorination of Hexachloro-1,3-butadiene in Rhine Sediment and Model Systems with Hydroxocobalamin | Bosma et al., 1994 | Link |
