Compound degraded:Toxaphene
General Description (About POP compound)
Toxaphene is made by reacting chlorine gas with a substance called camphene. The resulting product (toxaphene) is a mixture of hundreds of different chlorinated camphenes and other, closely related chlorinated terpenes. Toxaphene was one of the most heavily used pesticides in the United States in the 1970s and early 1980s. It was used primarily to control insect pests on cotton and other crops in the southern United States. Other uses included controlling insect pests on livestock and killing unwanted fish in lakes.
Biodegradation pathway
Publications
| Abstract | Title | Authors | Article Link |
|---|---|---|---|
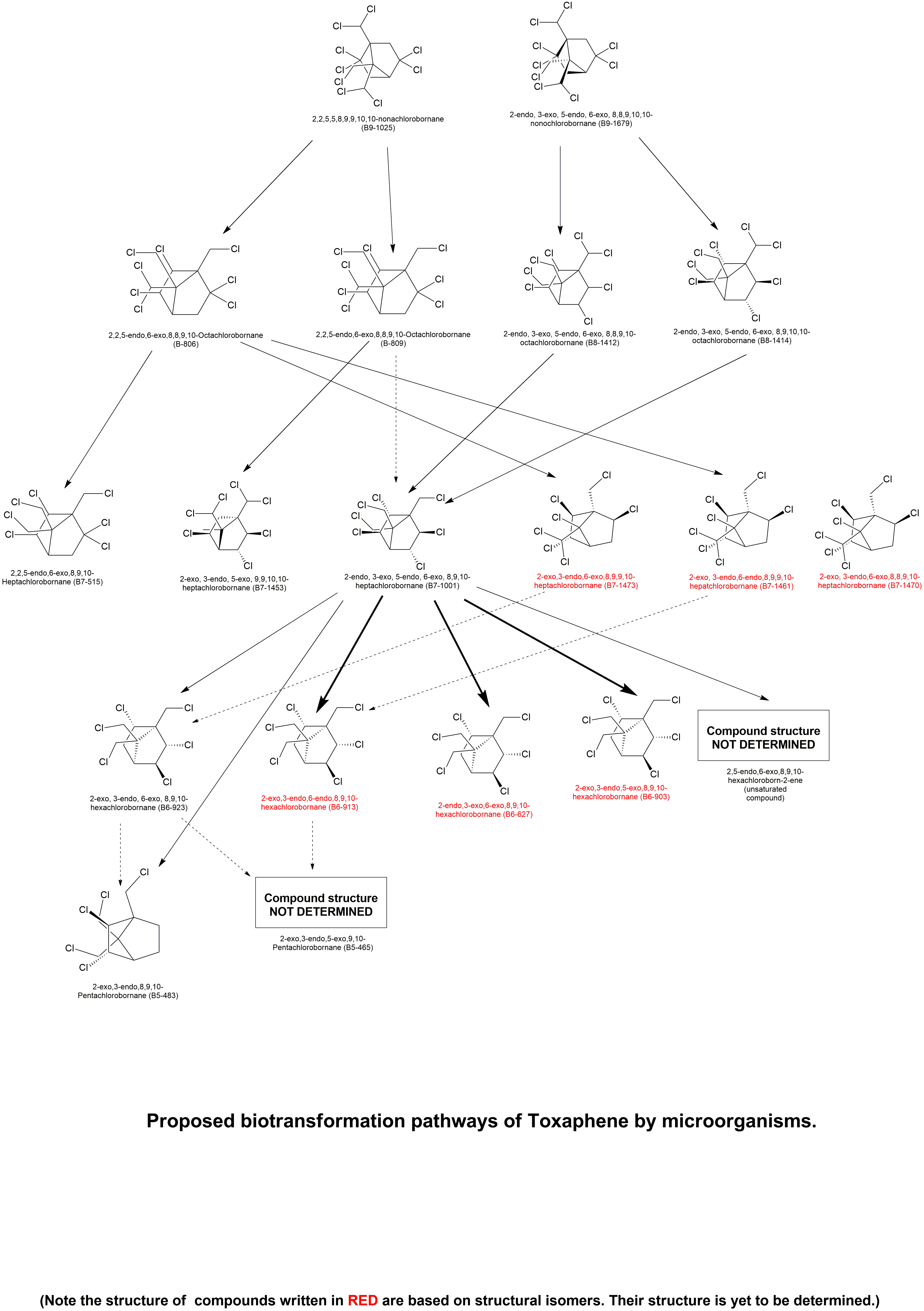
| Technical toxaphene (Melipax) and the single compounds of technical toxaphene (CTTs) 2,2,5-endo, 6-exo, 8,8,9,10- octachlorobornane (B8-806), 2,2,5-endo, 6-exo, 8,9,9,10-octachlorobornane (B8-809), 2,2,5,5,8,9,9,10,10-nonachlorobornane (B9- 1025), 2-endo, 3-exo, 5-endo, 6-exo, 8,8,9,10,10-nonochlorobornane (B9-1679), 2-endo, 3-exo, 5-endo, 6-exo, 8,9,10,10-octachlorobornane (B8-1414), 2-endo, 3-exo, 5-endo, 6-exo, 8,8,9,10-octachlorobornane (B8-1412), and 2-exo, 3-endo, 5-exo, 9,9,10,10-heptachlorobornane (B7-1453) were treated with suspensions of the anaerobic bacterium Dehalospirillum multivorans. After 7 d, more than 50% of technical toxaphene was transformed, and the relative amount of early eluting CTTs increased. After 16 d, only 2-exo, 3-endo, 6-exo, 8,9,10-hexachlorobornane (B6-923), 2-endo, 3-exo, 5-endo, 6-exo, 8,9,10-heptachlorobornane (B7-1001), and a few minor penta- and hexachloro-CTTs were detected in the samples. The result of the transformation was comparable with observations in naturally contaminated sediments and soil. However, the performance with D. multivorans was more simple and reproducible, as well as faster, than use of soil, sediment, or anaerobic sewage sludge. In agreement with reports in the literature, reductive dechlorination at geminal chlorine atoms (gem-Cls) was found to be the major CTT transformation pathway. Experiments conducted with CTTs and gem-Cls at both primary and secondary carbons clarified that the initial Cl -> H substitution takes place at the secondary carbon C2. Furthermore, the 2-endo-Cl position was preferably substituted with hydrogen. In the case of B8-806, the dechlorination at the secondary carbon C2 was approximately 20-fold faster than the subsequent, slow reduction at the primary carbon C8. The three different formerly unknown heptachloro-CTTs, 2-exo, 3-endo, 6-exo, 8,9,9,10-heptachlorobornane (B7-1473), 2-exo, 3-endo, 6-endo, 8,9,9,10-hepatchlorobornane (B7-1461), and 2-exo, 3-endo, 6-exo, 8,8,9,10-heptachlorobornane (B7-1470) were found as intermediates of the B8-806/809 transformation. Treatment of B9-1679 with D. multivorans indicated that gem-Cls on the bridge (C8 and C9) are dechlorinated faster than gem-Cls on the bridgehead (C10). | Anaerobic transformation of compounds of technical toxaphene. I. Regiospecific reaction of chlorobornanes with geminal chlorine atoms | Ruppe et al., 2009 | Link |
| Enterobacter sp. strain D1 is a facultative anaerobic, Gram-negative heterotrophic bacterium isolated from toxaphene-contaminated soil. This organism was identified and characterized through phylogenetic and taxonomic studies. Based on 16S rDNA analysis, the strain D1 was clustered closely with the species Enterobacter cloacae subsp. dissolvens (LMG 2683) and E. cloacae (ATCC 13047T). Strain D1 resembled these E. cloacae strains with respect to various biochemical and nutritional characteristics, but also exhibited differences. Moreover, strain D1 is able to grow and survive with toxaphene supplied in the medium in the range 3–96 mg/L. Amongst the chemical components of toxaphene, octachlorocamphenes, nonachlorobornanes and decachlorobornanes were seen to be rapidly metabolized, although levels of hexachlorocamphenes and heptachlorobornanes were found to be slowly degraded, and subsequently accumulated during the last stage of the cultivation. | A toxaphene-degrading bacterium related to Enterobacter cloacae, strain D1 isolated from aged contaminated soil in Nicaragua | Lacayo-Romero et al., 2005 | Link |
| The white-rot fungus Bjerkandera sp. strain BOL13 was capable of degrading toxaphene when supplied with wood chips, wheat husk or cane molasses as cosubstrates in batch culture experiments. Approximately 85% of toxaphene was removed when wheat husk was the main substrate. The production of lignin peroxidase was only stimulated when wheat husk was present in the liquid medium. Although xylanase was always detected, wheat husk supported the highest xylanase production. A negligible amount of ?-glucosidase and cellulase were found in the batch culture medium. To the best of our knowledge, this is the first reported case of toxaphene degradation by white-rot fungi. | Degradation of toxaphene by Bjerkandera sp. strain BOL13 using waste biomass as a cosubstrate | Lacayo-Romero et al., 2006 | Link |
| The major toxaphene metabolites in sediment and soils (2-exo,3-endo,6-exo,8,9,10-hexachlorobornane [B6-923] and2-endo,3-exo,5-endo,6-exo,8,9,10-heptachlorobornane [B7-1001]) were incubated with the isolated gram-negative bacterium Dehalospirillum multivorans. Within 14 d, biotransformation of B7-1001 was nearly quantitative, resulting in two penta- and six hex-achlorobornanes, as well as one unsaturated hexachloro compound of technical toxaphene. The major transformation product (˜50% of all metabolites) was identified as 2-exo,3-endo,5-exo,8,9,10-hexachlorobornane (B6-903). Abiotic dehydrochlorination of B7-1001 with methanolic KOH resulted in the formation and subsequent identification of the lone unsaturated compound as 2,5-endo,6-exo,8,9,10-hexachloroborn-2-ene. Thus, dehydrochlorination was found to be a minor process of the anaerobic transformation of toxaphene. Biotransformation of 70% of amended B6-923 within 14 d demonstrated that reductive dechlorination was not exclusively associated with geminal Cl atoms, as previously suggested. Three pentachlorobornanes were identified as transformation products, one of which was identical with a transformation product of B7-1001. This commonality unequivocally proves this metabolite to be 2-exo,3-endo,8,9,10-pentachlorobornane. Fifteen previously unknown metabolites of B6-923, B7-1001, and other toxaphene compounds identified in this study were detected in sediment from Lake Ontario (Canada), underscoring the importance of microbial toxaphene transformation in natural, aquatic environments. | Anaerobic transformation of compounds of technical toxaphene. 2. Fate of compounds lacking geminal chlorine atoms | Ruppe et al., 2009 | Link |
